What to know about the spring COVID booster
U.S. recommends shot for those over 65 or immunocompromised as a new phase of vaccine policy starts.
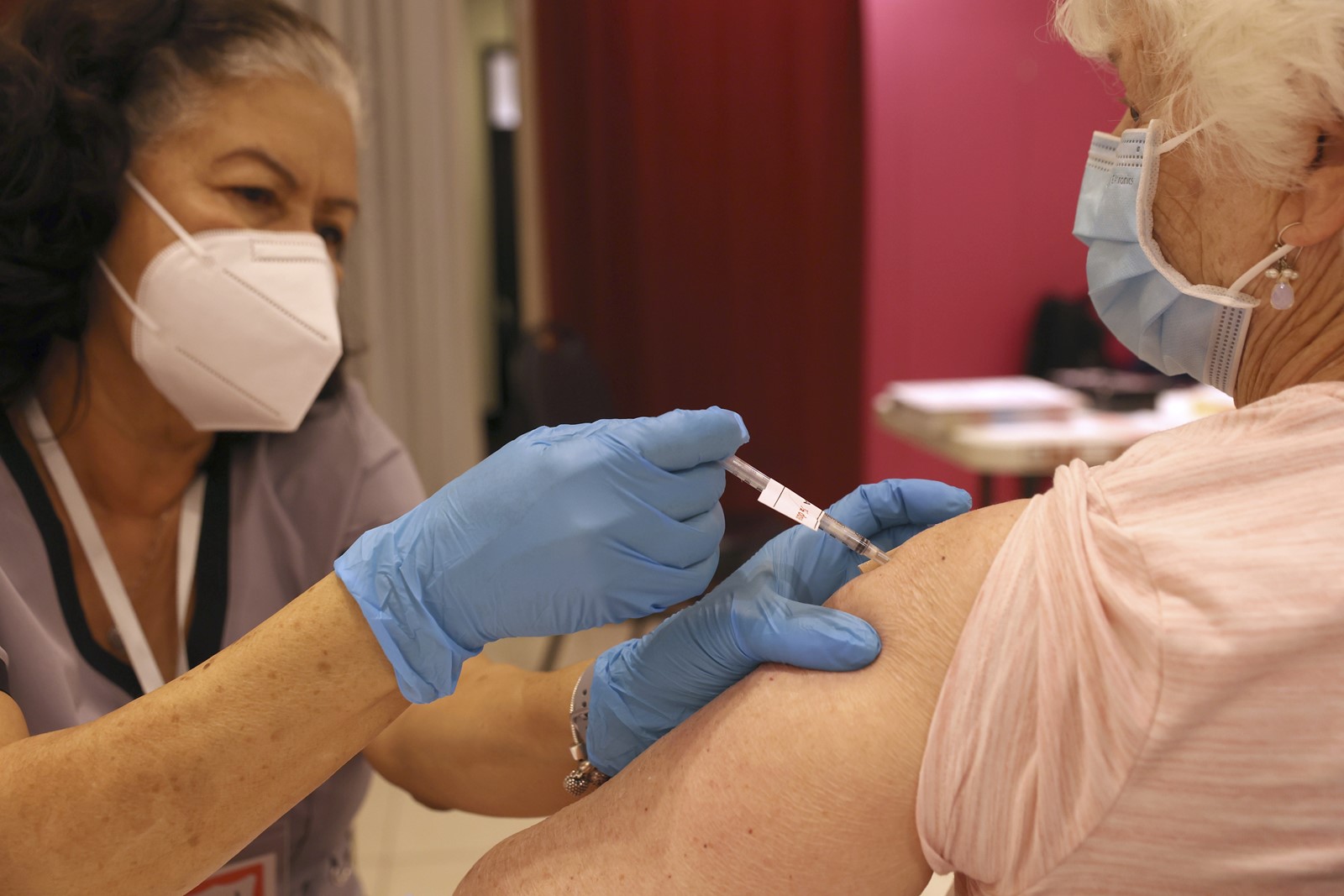
Those who are at least 65 years old can get another dose, according to the CDC. Immunocompromised individuals are eligible too.
According to language the FDA unveiled Tuesday, people 65 and older may receive one additional dose of the updated booster at least four months following their initial updated booster dose.
The agency also said that most immunocomprised people may receive an additional booster dose at least two months following their first updated booster dose. Such individuals, the FDA said, can also receive additional future doses at the discretion of their healthcare providers.
The CDC said it is recommending allowing the additional updated booster shot, which will “allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 doses.” In other words, seniors and immunocompromised people who are eager for the additional booster are free to get it, but the CDC is not necessarily urging those groups to get the shot with the same urgency as it did for most people last year.
Los Angeles County Public Health Director Barbara Ferrer said the CDC has been pretty clear that for most healthy people under age 65 who have already received the updated booster, “you’ve got really decent protection.”
“But I think for those people over 65, those people who are immunocompromised, it may be helpful to go ahead and get that second bivalent booster dose,” Ferrer said.
Another factor to consider: having plans to travel, which exposes people to more risk of infection.
Some doctors suggest older patients get the additional booster. Dr. Peter Chin-Hong, a UC San Francisco infectious diseases expert, said, “The older you are, the more important it is.”
“The bottom line is I would advise them to get it if you’re older than 65,” he said. “We know that antibodies wane at the three- to four-month mark [after a shot], and they wane the most in those who are older than 65.”
Aside from the above groups, most people who have already gotten the updated bivalent booster are not eligible for another dose. However, those who haven’t gotten the first shot are free to roll up their sleeves.
The CDC recommended that everyone 6 and older get the updated COVID-19 shot — regardless of whether they had completed their primary vaccination series. That means if you haven’t gotten a COVID-19 booster since September, or if you are unvaccinated, the CDC is recommending you get the updated vaccination now.
“For young children, multiple doses will continue to be recommended and vary by age, vaccine and which vaccines were previously received,” the CDC said.
Wednesday’s action means that people who are getting vaccinated for the first time need only one shot of the updated Pfizer-BioNTech or Moderna doses to be considered up-to-date on their COVID-19 vaccinations. Officials said they are ending the original two-dose monovalent regimens of those vaccine brands, which were designed against the ancestral version of the coronavirus and are now considered outdated.
Existing CDC recommendations on use of the monovalent vaccinations made by Novavax and Johnson & Johnson remain in place.
On Tuesday, the FDA authorized the following updated vaccine regimens for children younger than 6:
Eligible Californians will be able to schedule an appointment either directly through their healthcare provider or by using the state’s online platform, MyTurn.ca.gov.
Vaccines remain free even after recent moves to lift COVID-19 emergency declarations at both the state and federal levels.
Evidence has accumulated that those who have gotten the updated booster are better protected against hospitalization and death, Ferrer said. The benefit is particularly pronounced among older individuals.
“Available data continue to demonstrate that vaccines prevent the most serious outcomes of COVID-19, which are severe illness, hospitalization and death,” Marks said.
COVID-19 has remained the leading infectious cause of death in L.A. County, and can be especially dangerous for older people who aren’t up-to-date on their vaccinations and boosters, even if they’ve been previously infected and recovered. For the most recent weekly period available, 59 COVID-19 deaths were reported countywide.
According to a recent analysis, among seniors age 65 to 79 in L.A. County, those who got the updated booster had one-tenth the risk of being hospitalized compared with those who are unvaccinated, and roughly half the risk of being hospitalized compared with those who are vaccinated but haven’t received the bivalent booster.
Among this age group, those who got the updated booster were about one-tenth as likely to die from COVID-19 compared with unvaccinated people — and roughly half as likely to die compared with vaccinated people who hadn’t received the updated booster.
While no single health intervention provides absolute protection against infection, experts say getting vaccinated provides some benefit in that regard. That could prove useful given the emergence of another coronavirus Omicron subvariant, XBB.1.16, which some experts say could plausibly lead to another bump in cases in the coming months. There are anecdotes from India suggesting this particular strain is behind a number of reports of “COVID eye” — also known as pink eye or conjunctivitis — especially among children.
Still, the latest subvariant has not been associated with increased risk of severe illness.
The bivalent shots are updated versions of the original COVID-19 vaccine shots that first became widely available in September.
Regardless of the brand, the bivalent booster is formulated to protect against the hyperinfectious family of Omicron coronavirus subvariants that have dominated the nation for more than a year.
They were designed with BA.4 and BA.5 in mind, as those subvariants rose to prominence last spring and summer. But officials say they continue to work well against strains that have subsequently emerged, such as XBB and XBB.1.5.
The original formulations of both the Pfizer-BioNTech and Moderna vaccines were designed against only the ancestral version of the coronavirus, rather than the heavily mutated versions in circulation today.
“At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines, and the agency believes that this approach will help encourage future vaccination,” Marks said.
Don’t expect anything like the early days of the vaccine rollout — when people braved lengthy lines or hit the road in desperate search for shots. According to the California Department of Public Health, only about one-fourth of eligible residents have gotten a bivalent booster in the seven months since it became available.
However, that number belies regional disparities. A recent Times data analysis found that as of the end of March, nearly 35% of eligible residents in the San Francisco Bay Area had gotten the bivalent booster, compared with 23% in Southern California and only 16% in the San Joaquin Valley.


